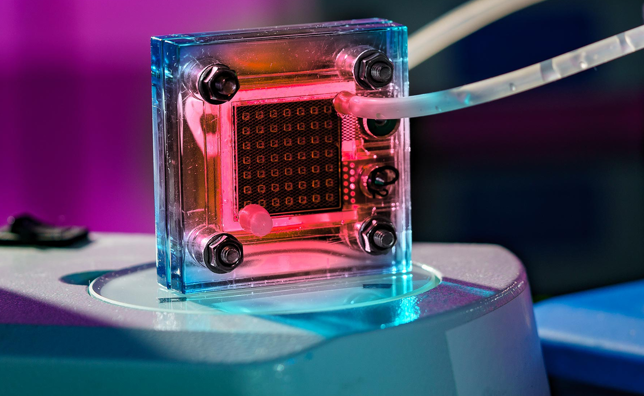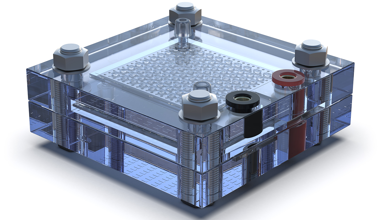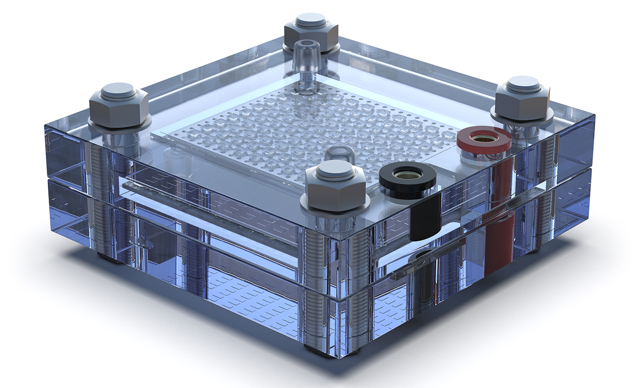
Fuel cells offer a multitude of advantages. They generate electricity with minimal pollution and zero greenhouse gas emissions, making them a valuable ally in the battle against climate change. Their quiet and efficient operation positions them as top performers in transportation, stationary power, and various electronic devices.
These innovative devices harness cleaner and more efficient energy by directly converting the chemical energy of hydrogen into electricity, presenting a greener alternative to traditional fuel combustion. Crucial to hydrogen fuel cell efficiency is the production of specialized membranes, particularly those designed for proton exchange. Ongoing research endeavors aim to create eco-friendly, high-performance, and cost-effective membranes, propelling us toward a sustainable energy future driven by the promise of fuel cells.
Fuel cell membranes play a crucial role in enabling proton exchange reactions, conducting protons while effectively blocking electrons to maintain power balance. Thin proton-exchange membrane (PEM) layers are crafted through techniques like solution casting and electrospinning.
One widely adopted material is Nafion, a perfluorosulfonic acid polymer, known for its exceptional proton conductivity and stability. However, ongoing research is dedicated to discovering more environmentally friendly and cost-effective alternatives. The production of these membranes necessitates stringent quality control to ensure uniformity and consistent proton conductivity, vital in advancing the field of clean energy.
What exactly is CCM, or "Catalyst-Coated Membrane," in the context of fuel cells?
CCM consists of three integral components:
1. Membrane: This thin polymer, often proton-conducting, such as Nafion, allows protons to transfer while effectively blocking electrons and gas molecules.
2. Anode Catalyst Layer: Coated with a catalyst, like platinum nanoparticles, it facilitates the conversion of hydrogen into protons and electrons.
3. Cathode Catalyst Layer: Another catalyst-coated layer, frequently platinum-based, enables the reduction of oxygen by combining it with protons and electrons to produce water.
CCM serves a critical role in fuel cells by facilitating electrochemical reactions, proton transport, and the generation of electricity. Proper CCM design and manufacturing are pivotal factors influencing fuel cell performance and efficiency.